Phesi has published its latest annual analysis of global clinical development activity, based on 65,892 recruiting clinical trials captured within its AI-driven Trial Accelerator platform. The 2025 findings keep breast cancer (across subtypes) in the number one position for the fifth consecutive year, followed by solid tumours, stroke, prostate cancer and non-small cell lung cancer (NSCLC).
Phase II trial attrition shows improvement, but remains elevated
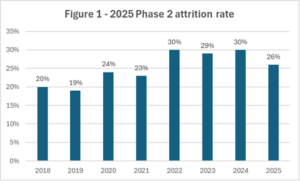
The report indicates early signs of post-pandemic recovery in trial continuation, with the Phase II termination rate falling to 26% in 2025. While this marks a four-year low, the level remains above pre-Covid baselines and continues to represent a major efficiency challenge for sponsors and programme teams.
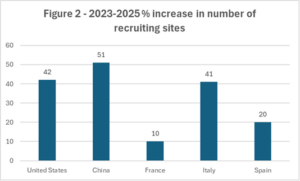
Geographically, the United States continues to host the largest number of recruiting clinical trial investigator sites overall and across the five leading disease areas. China ranks second across most of the top five indications and recorded the strongest growth in recruiting investigator sites between 2023 and 2025, increasing by 51% compared with 42% for the United States. France, Italy and Spain complete the top five countries for recruiting sites.
Implications for sponsors: capacity, representation, and data-led site strategy
Commenting on the findings, Dr Gen Li, founder and president of Phesi, said: “While it is positive to see trial attrition rates fall, a quarter of Phase II trials ending early is still unacceptably high.”
He added that sponsors are contending with macroeconomic constraints, pricing challenges, and increasing activity in regions such as China, alongside renewed emphasis from regulators on country-specific representation. In practical terms, this can lead to saturation at well-known, high-profile investigator sites—strengthening the case for systematically identifying additional sites with capacity and prior evidence of high-quality data delivery.
Obesity remains just outside the top five as GLP-1 research broadens
Phesi’s analysis places obesity in sixth position in 2025, narrowly missing the top five. The report notes expanding research interest around GLP-1 use across a wide range of diseases, reflecting obesity’s role as a comorbidity that can influence study parameters, dosing approaches and endpoints.
Read the full report
The full “Top Five Studied Diseases in 2025” report is available from Phesi here: Global Data Analysis of Clinical Development: Top Five Studied Diseases in 2025.
More information on Phesi’s Trial Accelerator platform is available here: Trial Accelerator.
Read more from the Pharma world here.